Mette Soendergaard • June 24, 2022
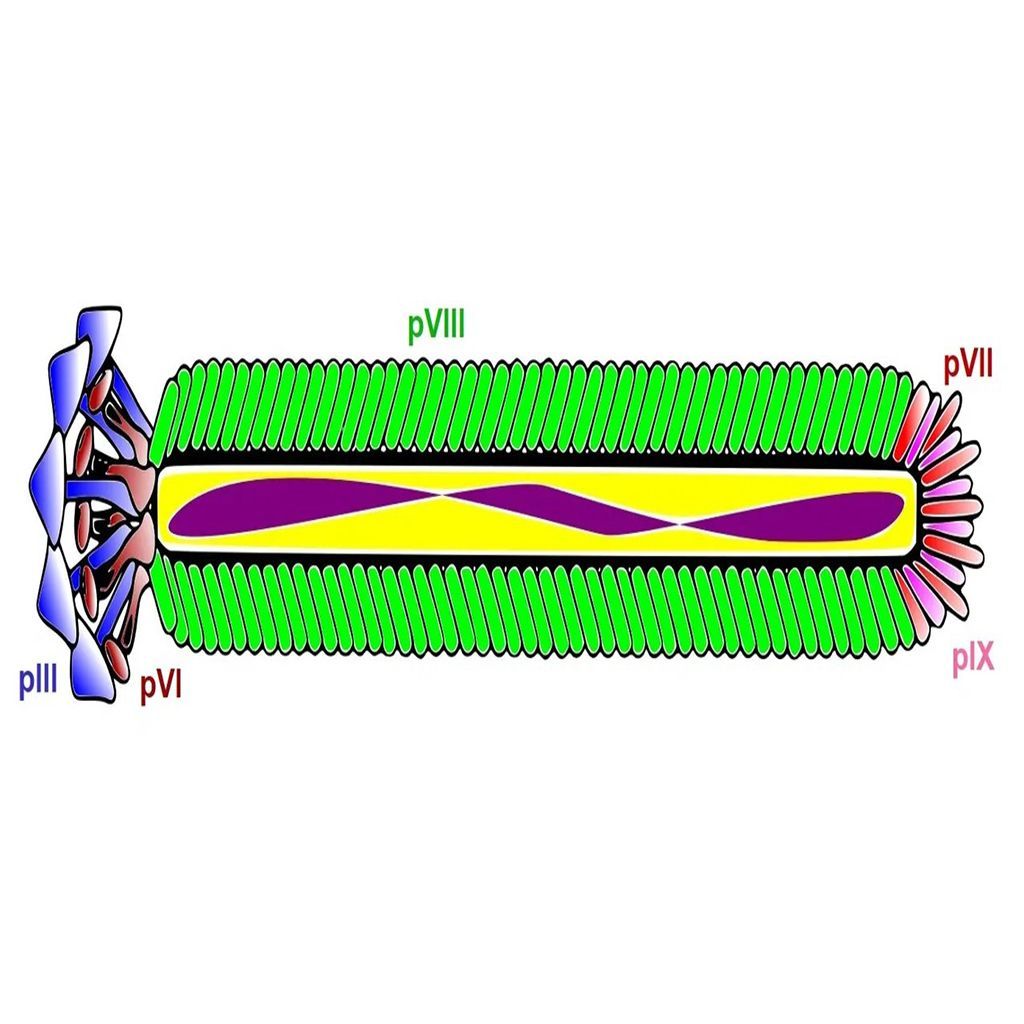
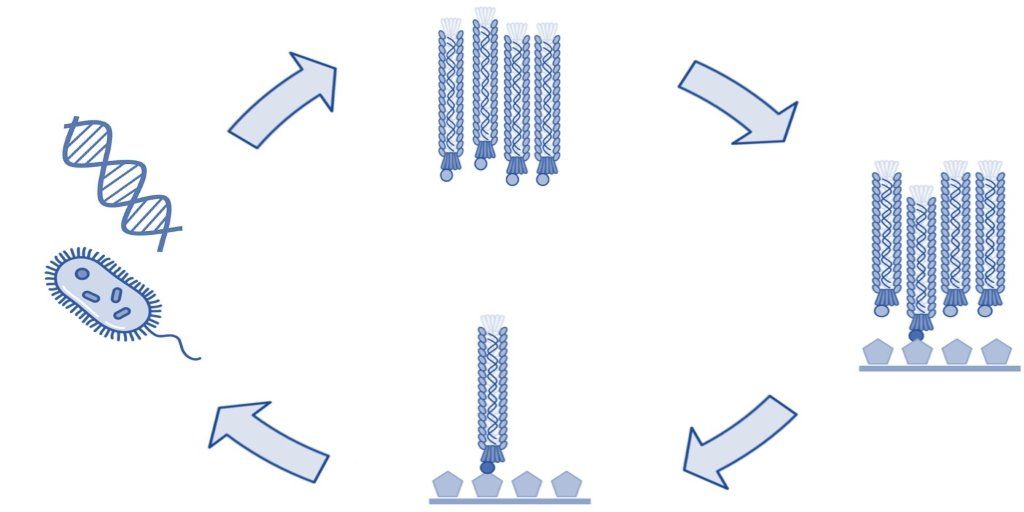
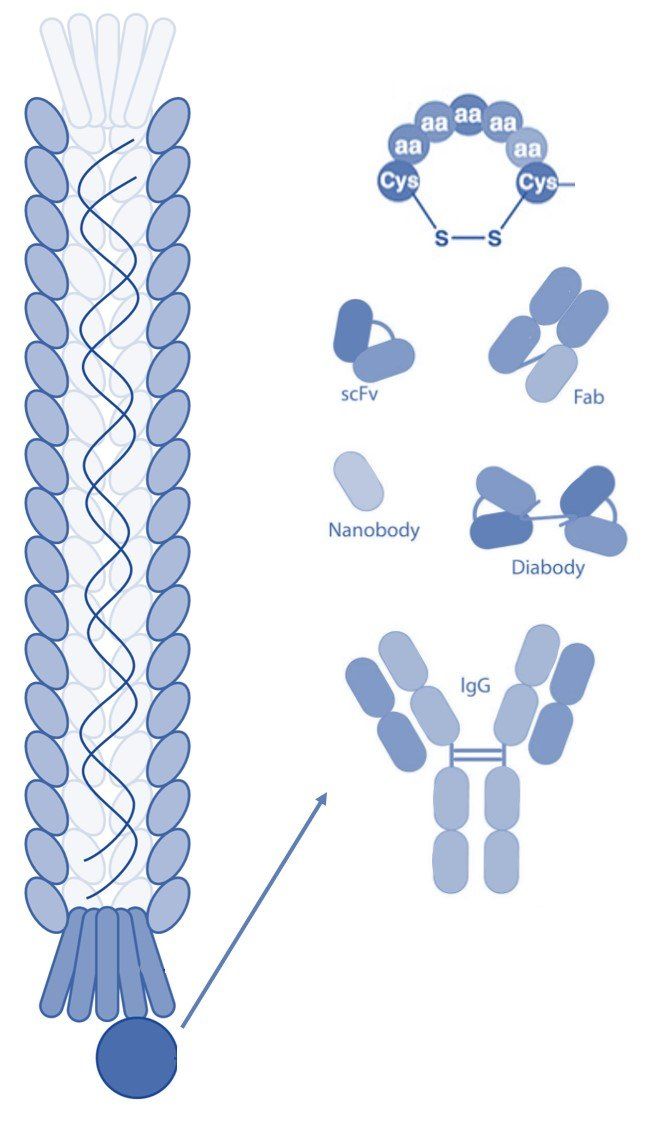
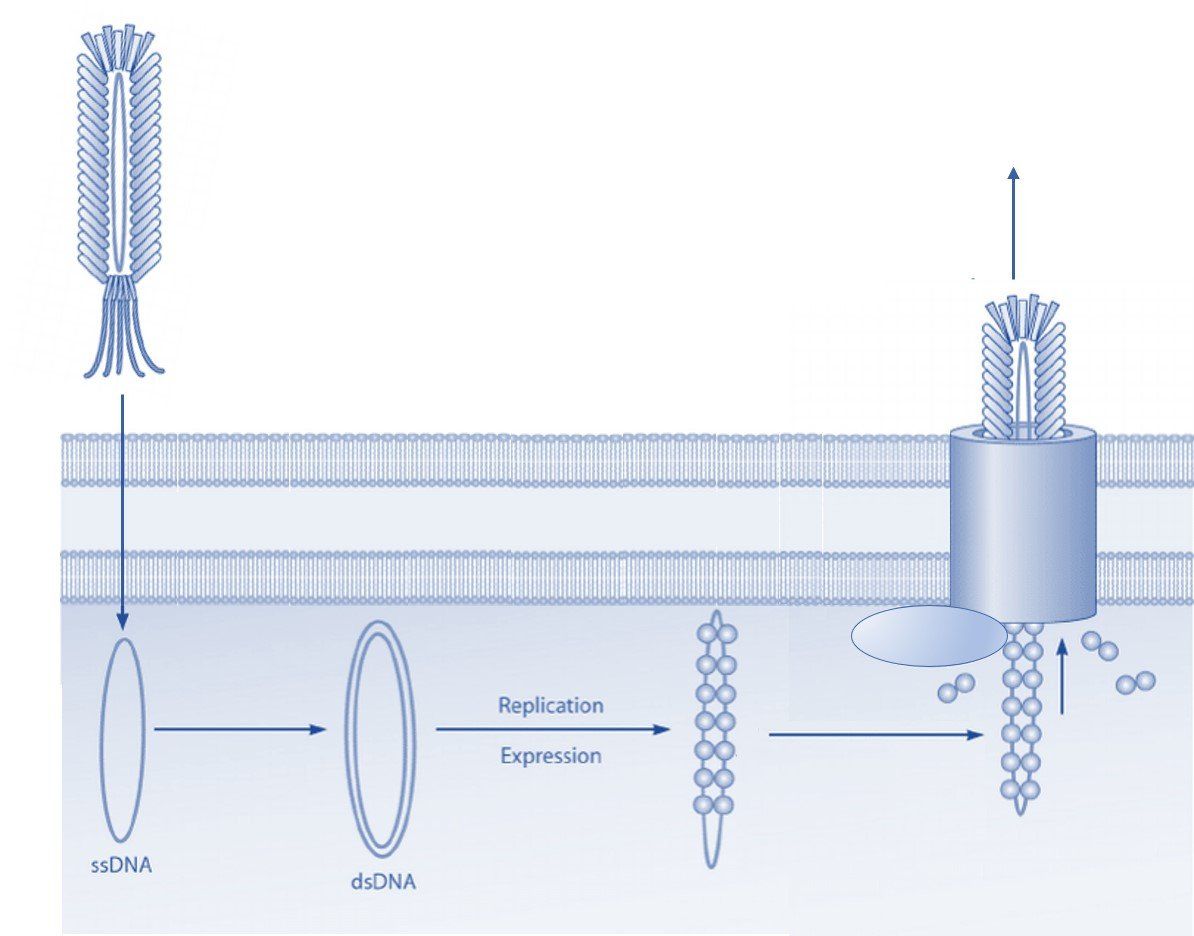
The structure of the filamentous phage offers many opportunities for display of polypeptides (peptides, antibodies, antibody fragments, nanobodies, etc.) onto various surface proteins. While phage display technology utilizing the minor coat protein III (pIII) and coat protein VIII (pVIII) are the most common, each type of phage display offers its own advantages.
pIII
Coat protein III interacts with the E. coli pili to initiate infection of the host cell. Despite the protein's involvement in infection, display of peptides is permitted without significantly affecting the lifecycle of the filamentous bacteriophages [1]. This property is important, since maintenance of infection rates ensures propagation of each clone in the phage display library, thereby avoiding selection bias. However, display of large polypeptides can substantially affect the infectivity rate and thus skew the selection results. Coat protein III is by far the most common system to display peptides and can be utilized without substantially changing the properties of the bacteriophage coat proteins.
pVIII
Coat protein VIII is the main coat protein of the filamentous phage of the Ff class and is expressed in over 2000 copies along the phage virion [2]. Typically, pVIII display is carried out utilizing approximately 10% of the coat proteins resulting in expression of about 200-300 copies of the foreign polypeptide. Expression of such a large number of peptide sequences can have advantages in certain types of selection techniques including those with a polyvalent target. Display on pVIII can also be carried out at lower expression rates and even monovalently to facilitate selection of high affinity ligands. Several peptide libraries have been made an successfully used in in vitro selections, amongst others. However, expression of large polypeptides, such as in an antibody library, is often difficult to achieve due to severe impairment of virion release during the phage lifecycle [3, 4, 5].
pVI
Coat protein VI is located at the distal end of the phage virion next to pIII. Display onto pVI is less common, but has been used to express larger interest proteins at both the N- and C-termini. Specifically, filamentous phage has been used to create cDNA libraries that offer advantages compared to similar methods (yeast-2-hybrid) due to the ease of propagation of the viral particles. While phage display selections using pVI are less common than those utilizing pIII and pVIII, pVI expression offers several advantages. This is especially true when creating open-reading frame libraries and studying protein-molecular interactions [6].
pVII
Coat protein VII is located at the opposite tip of the phage virion relative to pIII and pVI. Display on pVII is far less common than pIII and pVIII. However, in recent years pVII expression has received more attention specifically to display antibodies and antibody fragments for drug discovery. pVII display may also be used in combination with other types of display to facilitate easy post-screening analysis and tagging of the phage surface [7].
pIX
Display on coat protein IX has been greatly improved in recent years by utilizing various phagemid systems and DNA sequences to modify signal peptides. The display levels of foreign polypeptides on pIX tend to be lower compared to other systems, especially those that use pIII. However, the low levels of expression increase the probability of monovalent display that often facilitates selection of high-affinity ligands [7].
Summary
As a laboratory technique, phage display imparts the choice of using different coat proteins to display of various types of polypeptides. Due to the ease of expression, pIII and pVIII are the most commonly used display systems. However, pVI, pVII, and IX each offer their own advantages and display using these systems is becoming more popular.
References
[1] Sidhu SS, Weiss GA, Wells JA. High copy display of large proteins on phage for functional selections. J Mol Biol. 2000 Feb 18;296(2):487-95. doi: 10.1006/jmbi.1999.3465. PMID: 10669603.
[2] Malik P, Terry TD, Bellintani F, Perham RN. Factors limiting display of foreign peptides on the major coat protein of filamentous bacteriophage capsids and a potential role for leader peptidase. FEBS Lett. 1998 Oct 2;436(2):263-6. doi: 10.1016/s0014-5793(98)01140-5. PMID: 9781692.
[3] Jończyk E, Kłak M, Międzybrodzki R, Górski A. The influence of external factors on bacteriophages--review. Folia Microbiol (Praha). 2011 May;56(3):191-200. doi: 10.1007/s12223-011-0039-8. Epub 2011 May 31. PMID: 21625877; PMCID: PMC3131515.
[4] Marvin DA. Filamentous phage structure, infection and assembly. Curr Opin Struct Biol. 1998 Apr;8(2):150-8. doi: 10.1016/s0959-440x(98)80032-8. PMID: 9631287.
[5] Bauer M, Smith GP. Filamentous phage morphogenetic signal sequence and orientation of DNA in the virion and gene-V protein complex. Virology. 1988 Nov;167(1):166-75. doi: 10.1016/0042-6822(88)90066-9. PMID: 3263730.
[6] Jespers LS, Messens JH, De Keyser A, Eeckhout D, Van den Brande I, Gansemans YG, Lauwereys MJ, Vlasuk GP, Stanssens PE. Surface expression and ligand-based selection of cDNAs fused to filamentous phage gene VI. Biotechnology (N Y). 1995 Apr;13(4):378-82. doi: 10.1038/nbt0495-378. PMID: 9634780.
[7] Løset GÅ, Roos N, Bogen B, Sandlie I. Expanding the versatility of phage display II: improved affinity selection of folded domains on protein VII and IX of the filamentous phage. PLoS One. 2011 Feb 24;6(2):e17433. doi: 10.1371/journal.pone.0017433. PMID: 21390283; PMCID: PMC3044770.
[8] Modified from By J3D3 - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=30612417